COVID-19 testing: exploring antigen and molecular tests
Allegheny students have once again returned to on-campus classes amidst the COVID-19 pandemic, and with this return has come thousands of diagnostic tests.
Diagnostic testing for SARS-CoV-2, the virus that causes COVID-19, has become widely used especially in the United States which leads the world in number of tests and is third in the rate of testing, according to statista.com.
According to the FDA, sensitivity and specificity are used to access diagnostic testing.
Sensitivity is defined as the fraction of positive cases that the tests identifies as positive. A test with a high sensitivity will be good at successfully identifying what is known as true positives. A true positive test would be a correctly identified presence of what is being tested for, in this case SARS-CoV-2.
Specificity is defined as the fraction of negative cases that the test identifies as negative. Similar to specificity a test with high specificity will be good at identifying true negatives.
Tests with high sensitivity are good at detecting even small amounts of material and tend to have a high rate of false positives as long as the specificity is low. Tests with a high specificity are good at detecting the virus, but can produce a high rate of false negatives if the sensitivity is too low.
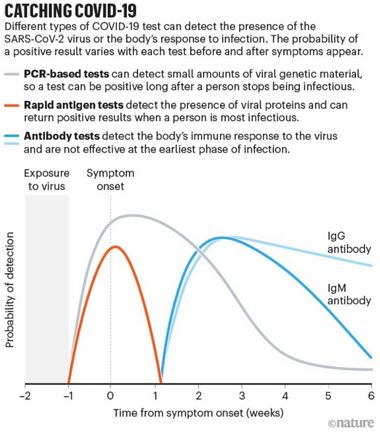
There are two types of diagnostic tests for the virus that causes COVID-19 according to the FDA: the molecular test and the antigen test. They both detect the virus, but in different ways.
An FDA overview explained that a molecular test detects the genetic material of the virus, often using polymerase chain reaction technology to amplify and detect the genetic sequence of the virus.
The advantage of this test is that it is highly specific and sensitive, although it takes hours to days for tests to be evaluated due to the technology and machinery needed, as well as the demand for this type of test.
The typical process for a molecular test begins with sample collection and then lab work. The lab work involves specific chemical mixes designed to extract genetic material which are combined with the sample, according to the FDA
After that, a machine is used to convert the RNA in the sample to DNA, and then makes millions of copies of that DNA sequence. If the SARS-CoV-2 genome is present, a reaction will take place in which the DNA bonds to the chemical mixture and produces a special type of light that is then detected by the machine.
The aforementioned overview also explained that an antigen test detects specific proteins present in the particles of SARS-CoV-2. These tests are what is often used in rapid tests. According to the CDC, they are considered to be generally less sensitive than molecular tests, but are still highly specific.
According to the FDA, some rapid tests are molecular, but the majority of rapid tests use the antigen method.
Antigen tests for COVID are similar to a test for strep throat at a doctor’s office. These tests were designed and used for the purpose of getting results rapidly, according to the FDA.
A difference between antigen and molecular testing is that antigen testing often happens at the point of care, which means the same place where the test is given. While rapid molecular testing has been developed, it is more common for these tests to be sent to a lab for testing.
The advantage of antigen tests is that it can give results quickly, so that those who are sick can be isolated, and that they are less expensive to conduct. According to the FDA, doctors may order a molecular test after a positive antigen test to confirm.
A study by Cochrane Library that evaluated the sensitivity and specificity of both antigen and molecular tests found that while both had similar specificity, the molecular test was significantly more sensitive. The study goes on to say that rapid, antigen tests can be used to inform the use of molecular tests. An example of this would be someone getting an antigen rapid test, testing positive, and then being retested to confirm that result. This would enable for the quick identification of potential positives with confirmatory tests.
For the spring semester, students were required to have a negative molecular test before returning to campus according to an email sent to students by Inspire Diagnostics, the company that Allegheny partnered with to test students.
Both types of tests have a place in the fight against the spread of COVID-19. When antigen tests were first approved the FDA acknowledged that while they may be less accurate in identifying the presence of COVID-19, there was a lot of potential for them to be mass produced and widely distributed, especially as more firms began to produce them.
Another branch of testing is to look for antibodies which detect if someone has developed an immune response to the virus. According to the CDC, this test is not useful for diagnostic purposes as it may take weeks for antibodies to develop.
Both tests contain tradeoffs, but when used in conjunction with each other they have the potential to inform medical decisions to help to prevent the spread of COVID.




