Professor provides first documentation of chemical interaction
In a journal article currently under review by the Journal of the American Chemical Society, Mark Ams, assistant professor of chemistry, explains an interaction that has never been documented before. The interaction demonstrates the strength of a chemical interaction that happens with molecules containing a flourine group above a ring structure, which is six carbons attached to each other.
Ams’ lab is currently studying the weak interactions that occur within one molecule. In the structure they use, called a torsion balance system, a long chain of carbons in the molecule fold in such a way that the end of the end is able to fold over the ring structure.
This torsion balance system is required to isolate the specific molecular force that they wish to study, a force that is so weak it is typically not able to be measured, according to Rosey Sheridan, ’15, who has worked on this project.
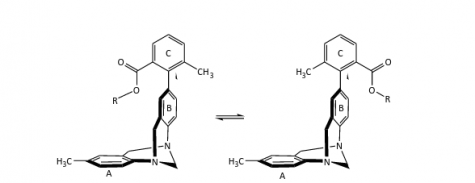
The molecule on the left depicts the folded conformation, which is sometimes lower energetically. The R-group interacts with the pi system above the ring labeled A.
“It’s not a very strong force at all, so if you tried to study it in systems where it’s usually present, you can’t. There’s all kinds of other things interfering. You can’t get a good measurement of it,” Sheridan said. “Our group uses these things called molecular torsion balances to basically isolate the interaction to quantify exactly how strong it is…The shape of the torsion balance kind of negates how all of those other forces affect the system.”
The major focus for the study is the role of fluorine, the most electronegative element, meaning that fluorine atoms draw electron density toward themselves more than atoms of other elements. The ring structure located on the other end of the molecule has a slightly negative charge above and below the ring itself, called a pi system.
When three fluorine molecules are bonded to a central carbon that is dangling above the carbon ring, these fluorines cause the central carbon to be slightly positive. This positive carbon then interacts with this slightly negative pi system.
“[Fluorine] pulls electron density towards itself and what we found is that, if you put enough fluorines out here, the fluorines are all pulling electron density towards themselves,” Ams said. “That leaves the carbon, which they’re bonded to, that’s what’s attracted to the pi system.”
Once the torsion balance is established as the molecule they are working with, Ams and the students in his lab are able to measure the strength of the interaction. When the molecule has this interaction, the molecule stays in what is called the folded conformation more often than it exists as the unfolded conformation. When there is an attractive force between the ends of the molecule, it is considered a lower energetic state, which the molecule prefers. The more often the molecule prefers the folded state, the stronger the intramolecular interaction is.
“If it prefers to have an attraction, it’ll stay there more,” Ams said. “We measure the population in which it likes to stay there and we can convert that to an attraction number, a real number and this is a very weak attraction. It’s hard to isolate but this molecule does it.”
While the interaction is rather weak, it can become a stronger interaction when many of these interactions occur within one system.
“That’s really important because [this] interaction is really present in proteins…so when the protein folds up, different amino acids can interact to form a lot of these really weak interactions, which even though they’re weak are very additive,” Sheridan said. “Over a thousand amino acids, they can be really formative in the way that a protein folds.”
Aside from the biological applications regarding protein folding, this research may potentially be relevant for the pharmaceutical industry, which has recently seen a prevalence of fluorine. According to Ams, three out of the 10 best-selling drugs in 2011 contained fluorine.
When a pharmaceutical company works on testing a new drug, many different versions of the same molecule must be tested to determine the efficacy and safety of the potential drug, but the testing of thousands of similar molecules can slow the process significantly, Ams believes.
“To find that drug that worked the best, a lot of times what these companies have to do in their [research and development] department is screen it and a couple other thousand of its buddies that are slightly different,” Ams said. “Make these molecules, test them in clinical trials or some other kind of trials, find which of those work the best and market that. Obviously, that takes lots of time and money.”
However, as chemists work to better understand the interactions within molecules, computer predictability becomes a more feasible future for chemistry and pharmaceuticals. This may only be realized with extensive work to fully understand even minor interactions, such as the work being done in Ams’ lab.
“One of the directions that people speculate chemistry and technology will be, will be in a place where we can rely on computers to accurately predict how molecules are going to behave without doing the back-breaking research in the laboratory,” Ams said. “Right now, if we want to study the way things behave, we have to get in there, do the chemistry, study it…But what if we had computer programming that can predict, accurately, this is the best structure?”
This is especially true in solvent systems, which the human body is. Most data on chemical interactions currently available is focused on gas phase interactions and these can change when the molecules interact in a liquid system when dissolved.
“Knowing how it’ll interact in different solvent systems is pretty important, just because most systems are going to be water-based, mixed with other things inside of it,” said Jake Patterson, ’17, who also works in Ams’ lab.
As the project continues, Patterson will be focusing on a tri-fluorinated carbon’s interaction with the pi system when dissolved in different types of solution. The students currently comping with Ams are also working on substituting different chemical structures to see how that affects the interaction with the pi system.
Regardless, the isolation of the weak forces between a tri-fluorinated carbon and the pi system represent a breakthrough that no other lab has yet quantified.
“Whenever we started seeing an affinity for folding when you had the fluorines stacked, we started talking about why that was and nobody’s really seen it before so that’s why [the Journal of the American Chemical Society] was so interested in it,” Patterson said. “Nobody has proposed this interaction before, nobody’s really had any evidence.”






